
Quality
In the company Medicamen we strive that socially responsible organizational culture and quality policy constitute the building block of future for all our employees.
With the introduction of the quality management system we assure a solid connection with our business partners, suppliers and satisfied buyer and end-users to build long-term cooperation and partnership. We strive to achieve the philosophy of continuous learning company. We run & target defined processes, measure their effectiveness and business performance.
Constant concern of all employees is that our products and services meet the needs and expectations of our customers throughout the life cycle. We establish a quality management system that enables continuous improvement of socially responsible organizational culture and its values. Development and manufacture of products is handled in accordance with the objectives and modern environmental protection policy requirements.
Quality Control
We have a modern and well-equipped Quality Control (QC) laboratory, which ensures that our products are pure, safe and effective and are released only after thorough analysis as per stringent specifications, methods and procedures developed according to international guidelines.
Our QC department has all necessary instruments for analysis of API, finished products, packaging and related materials used.

The QC department performs following activities:
RM/PM analysis
.
Finished Products analysis
RM/PM analysis
In-process Checks
Quality Control for API / PM, Finished Products & In-Process Control is as follows :
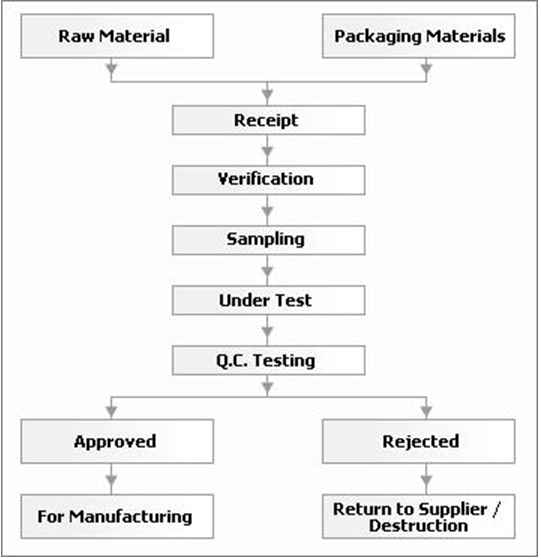
Flow Chart - RM / PM Inspection::
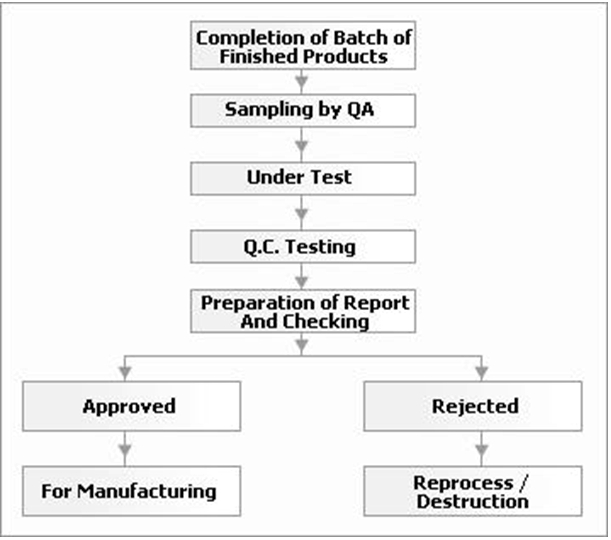
Flow Chart - Finished Products Inspections
Flow Chart - Inprocess Checks ::

The QC activities are managed through four sections
Instrumental Analysis and Finished Products
.
Wet Analysis Laboratory
Microbiological Testing Laboratory
Packaging Material -Testing Laboratory
Quality Improvement Plans :
| 1. Feedback received from the compliance team |
| 2. Proposals for corrective & preventive actions |
| 3. Annual products review |
| 4. Trend Analysis of various quality parameters for products, environment & water. |

Quality Assurance
Medicamen’s quality policy is mandated and supported by the Executive Management and coordinated by an independent Corporate Quality Assurance (CQA) Department.
Highlights :
| 1. Quality Assurance is independent of Manufacturing. |
| 2. In-Process quality is checked during manufacturing. |
| 3. Validation of facilities, equipments, process, products & cleaning as per Master Plan. |
| 4. Complaint Handling. |
| 5. Storage of quality record and control samples. |
| 6. Stability Studies. |
| 7. Registration Documents |
.
Documentation Control:
| 1. Controlled distribution and archiving of documents. |
| 2. Control of changes made by proper change control procedure. |
| 3. Approval of all documents |
Validation :
| 1. Preparation of validation plans for facility / equipments / process including cleaning. |
| 2. Approval of protocols for validation of facility / equipment / product / process. |
| 3. Team member for execution of validation of facility / equipment / product / process. |

Assuring Quality of Products :
| 1. cGMP training |
| 2. SOP compliance |
| 3. Audit of facility for compliance |
| 4. Line clearance |
| 5. In-process counter checks |
| 6. Critical sampling |
| 7. Record verification |
| 8. Release of batch for marketing |
| 9. Investigation of market complaints |
| 10. Stability of products |
